November 2025
During our last trip to Thailand, we went to the Plant-a-seed: café & bar. It is nowhere near a town, so they have space for a garden with a small pond. Kingfishers sometimes come to eat the fish in the pond, and one of them shed a bright blue-green feather. I brought the feather back to England so that I could try to photograph it. I decided to try a mixture of reflected light from my BH2-MA Brightfield Vertical Illuminator (with retroDIODE Obh10B LED illuminator) and normal transmitted light. I used a 45 mm LBD filter on the light output to try to match the colour of the LED. The MSPlan 10 0.30 ∞/- objective provided a suitable field of view, about 2.85 mm wide in my Canon EOS 5D Mark II. I used two coins to hold the feather fairly flat on a slide, and took a series of 29 images at .005 mm intervals to combine in Zerene Stacker (PMax).

Kingfisher feather (84 mm)
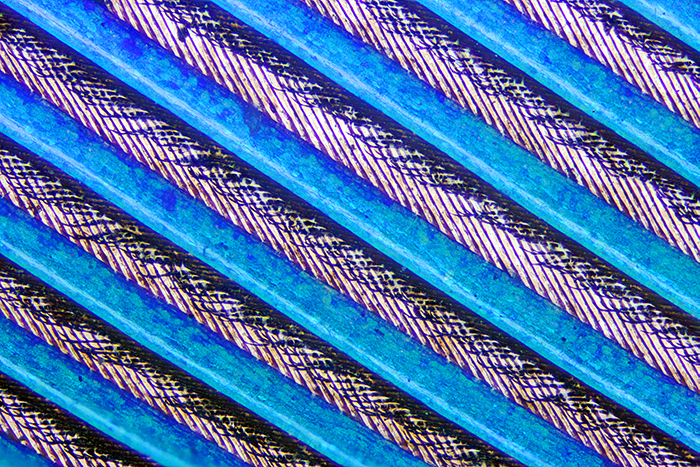
Kingfisher feather under the microscope (10× objective)
The blue colour comes from the barbs; the barbules and the rachis are dark brown. The common kingfisher in England is Alcedo atthis, and it also occurs in Thailand. Its feathers are semi-iridescent, a type of non-iridescent structural colouration. See Kingfisher feathers – colouration by pigments, spongy nanostructures and thin films by Doekele G. Stavenga et al. in Journal of Experimental Biology ((2011) 214 (23): 3960–3967.)
Diancui (tian-tsui or 点翠) was a traditional kingfisher feather decoration technique in China that involved cutting off the brown barbules and pasting the blue coloured barbs close together as part of artwork and jewelry. See Analytical investigation of the feather decoration technique of a seventeenth to eighteenth century Chinese imperial hanging screen by An Gu et al. in Heritage Science (July 2021 9(1):82).
October 2025
At the “Your go-to equipment” gossip meeting on Zoom in March, I showed the LED ring-light that I use with my stereo microscope and with macro lenses, and explained how I produce shadowless and dark-ground illumination. The theme of Quekex this year is “I made it or I modified it”, so I decided to use the same topic but with a PowerPoint show on my old Lenovo laptop. In March, I used small photos (700 px wide) from meeting reports, but this time I found the original photos and prepared larger images.
Click the arrows to move through the slides. Click the symbol at bottom right for a larger version.

My exhibit at Quekex
The shadowless illuminator works well with circuit boards, but I had not previously photographed one. I recently threw away an old analogue security camera, but I kept the sensor and the circular circuit board to which it was attached.
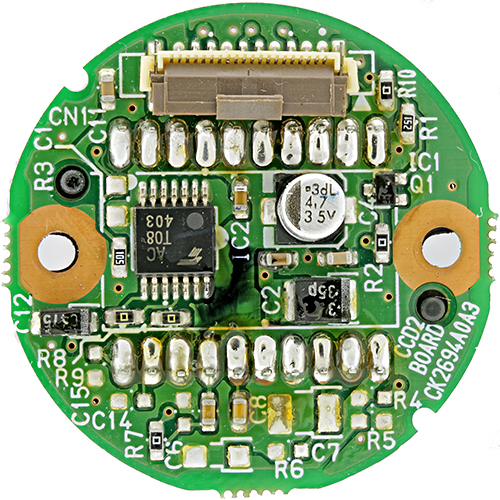
Sensor circuit board from video camera
I helped out on the Quekett stand at New Scientist Live for two very busy days. Other people provided the microscopes and specimens, so I only needed to take my camera. I managed the occasional cup of coffee, but there was no time to sit down and eat my sandwiches.

Talking to visitors at New Scientist Live [by Andy Chandler-Grevatt]
I helped out at the Quekett stand at the National Honey Show for all three days. The first day was so wet that I couldn’t take a microscope, but I managed my laptop and camera. The other two days, I also took my Olympus SZ4045 stereomicroscope and some specimens and slides.

My laptop and stereo at National Honey Show
Pam Hamer was showing slides of pollen and honeybee anatomy, so when I got home I photographed some similar slides to include in the meeting report. For the lily pollen slide, I used my Olympus SPlan 40 0.70 160/0.17 objective and took a set of 37 images at 0.001 mm steps that I combined in Zerene Stacker.
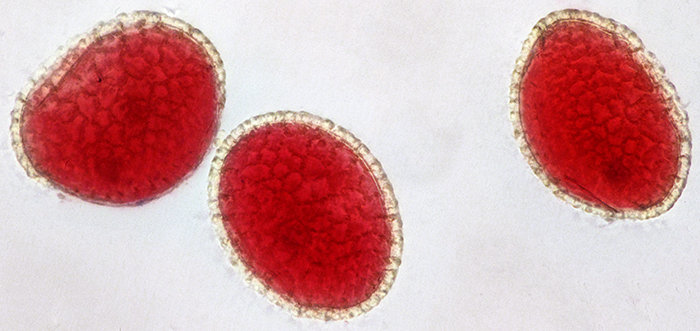
Lily pollen grains (40x objective, lengths 80, 83 and 90 µm)
For the honeybee head and third leg slides, I used my Canon EF-S 60mm 1:2.8 macro lens and EOS 600D, with the slides on a light box.
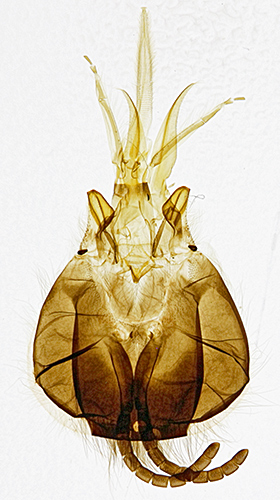
Head of honeybee

Third leg of honeybee
September 2025
I was looking forward to three outreach events this month, but there were no trains for the Grove Park Carnival, Wimbledon Common Open Day was cancelled because of bad weather, and we didn’t have enough volunteers for the AES Annual Exhibition. With no meeting reports to write, I had time to work on a PowerPoint show for Quekex next month. I also had time to add a row of year buttons at the top of my blogs, so that it is easier to move between them.
Kai and I noticed some small dark objects in slices of bread (not seeded). I thought they could be the start of mould, and Kai thought they were insects because she could see legs. The stereo microscope showed that we were both wrong, they were seeds that had been cut in half.

Seed in bread (2.4 mm)
I have always assumed that my LED ring-light would be no use with the Olympus 20 mm and 38 mm Auto Macro lenses because it would only shine light in a ring around a specimen, not onto it, but I decided to test it today. The 20 mm Zuiko Auto-Macro Lens seemed the best choice, and I started by attaching it to my Telescopic Auto Tube, only to discover that I could not stop it down. So I switched to the Auto Bellows, which has a lever to close down the automatic iris. I set the lens to f/4, and tried to clamp the LED ring-light on to the rubber focusing ring. At first it did not fit, because the ring-light fouled the front standard of the bellows, but a 14 mm Auto Extension Tube fixed that problem. With the Auto Bellows attached to my copying stand, a quick test showed that the light did illuminate a specimen about 20 mm from the front of the lens. So I set up my butchered BHTU stand so that I could adjust the focus in small steps and my Chuo goniometers so that I could tilt the specimen to the best angle.
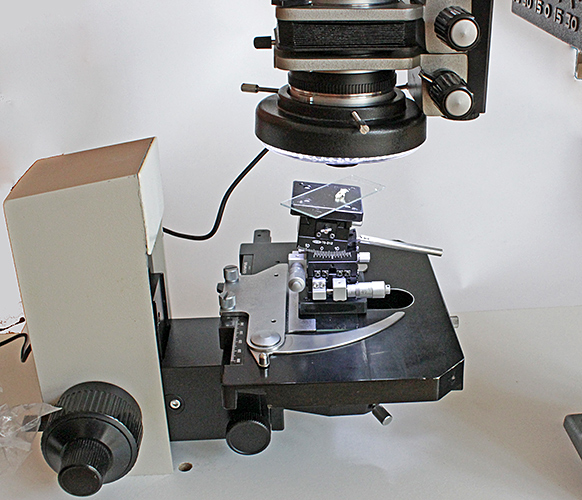
LED ring-light on Zuiko Auto-Macro 20 mm f/2
I used the lens at f/4 and took 16 images at 0.05 mm steps, for combining in Zerene Stacker PMax. I adjusted levels and brightness in Photoshop Elements 15.
August 2025
On Saturday 2nd August I took a few items to sell at Microscopium in Letchworth, and made enough to cover the cost of my train ticket. There were fewer sellers and not as many interesting items as usual, but it was a good opportunity to catch up with old friends.
On Saturday 9th August, I went on the Quekett excursion to Warnham Local Nature Reserve on the outskirts of Horsham. The specimens that we found in the samples included diatoms, desmids, rotifers, ciliates (including peritrichs), copepods, Cladocera, ostracods, mayfly nymphs, mites, gastrotrichs, testate amoebae, a leech, green hydra (Hydra viridissima) and algae.

Neil Henry sampling the Shelley pond
I went to the Microscopy Weekend at Down House on Saturday 16th to take photographs and help out. They were short of volunteers for Sunday 17th, so I went again, this time with my Olympus SZ4045 stereo microscope.

Olympus SZ4045 stereo microscope with 144-LED ring-light
I was based in the garden laboratory, and my specimens included flowers, fern sori, hollyhock seeds, poppy seed heads, lichen on small twigs, and a small mouldy apple, all collected from the garden. The poppy seeds looked interesting at maximum magnification under the stereo, so I took a seed head home to photograph. For the seed head, I used my shadowless illuminator based on an inverted LED ring-light and a white pudding basin, and this helped to illuminate the internal structure.

Seed head of a poppy (with shadowless illuminator)
For the poppy seeds, I used my Olympus BHT with a BH-RLA Brightfield/Darkfield Reflected Light Illuminator (set for darkfield), a Neo 5× objective and an FK 2.5× photo eyepiece. The seeds are very dark, and even with the lighting on maximum the image in EOS Utility on my computer screen was too dark to focus. I could just see enough through the eyepieces to focus, and I took 41 images at 0.020 mm steps for combining in Zerene Stacker (PMax). I had to adjust levels and brightness in Photoshop Elements 15 to show the surface detail.
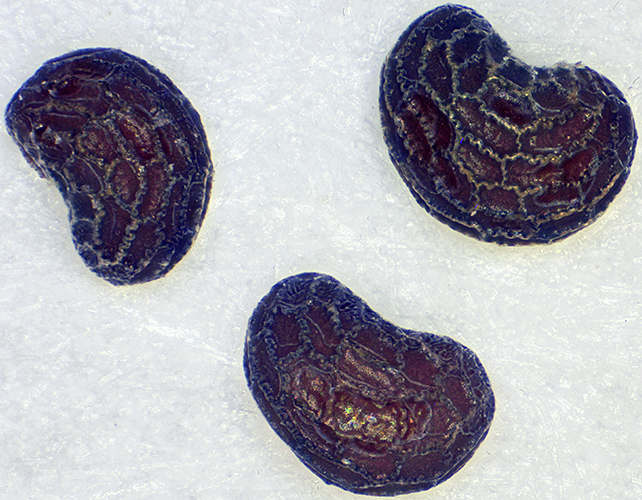
Poppy seeds (0.75 mm)
I have the original BH-LHM lamp house with a 6V 15W tungsten bulb, an alternative retroDIODE LED illuminator, and a BH2-MLSH lamp house with a 12V 50W tungsten halogen bulb, but none of them provide enough light for a dark specimen with reflected darkfield illumination.
We have recently planted some Portuguese laurels (Prunus lusitanica) in our back garden, and this was one of the species that Chris Thomas was using to demonstrate making leaf peels in Vida Rosa UV Resin. Chris talked me through the process, and I took the slide home to label it and photograph it. The peel is colourless so it was not easy to make it show up in a photo, but I found that diffuse illumination worked when the slide was raised above a black background.

Leaf peel of Portuguese laurel
To show the detail in the leaf peel, I used normal transmitted illumination on my BHT, with a 40× SPlan objective and a 2.5× NFK LD photo eyepiece. The peel was not flat, and I needed to take 34 images at .001 mm steps to get enough depth of field.
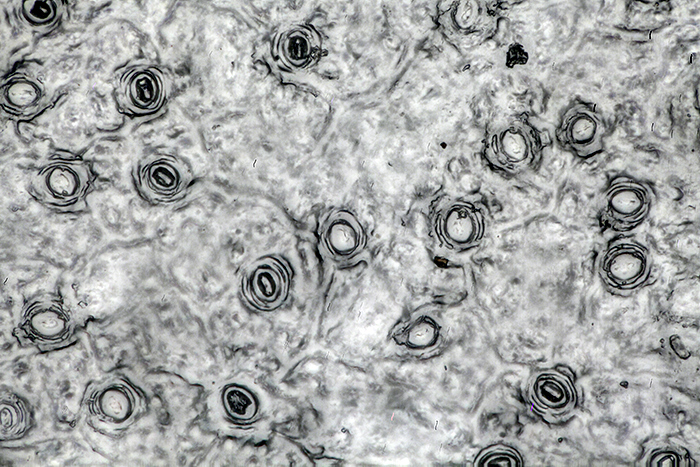
Leaf peel of Portuguese laurel (40× objective)
July 2025
Caterpillars of the peacock butterfly (Aglais io) used to be a familiar sight on stinging nettles (Urtica dioica), but I have not seen any for several years. These were feasting on nettles on the roadside verge behind our garden. I used my Canon EOS 600D with the EF-S 18–55 zoom lens.

Peacock caterpillars on stinging nettles
June 2025
On Saturday 21st June, I went on the Quekett excursion to Keston ponds. I went collecting with Nigel Ashby, and his specimens included copepods, cladocerans, ciliates, flagellates, desmids, bryozoans and rotifers.

Nigel Ashby collecting
May 2025
I went to the Natural History Museum for the “Stained sections“ gossip meeting and took some slides that I had acquired at Club meetings since joining the Quekett in 2011, including ones made by Quekett members Eric Impey, Ernie Ives, Colin Kirk, Eric Marson (NBS), Mike Smith and John Wells (Biosil), some axolotl sections described as “probably by Robin Wacker”, and some commercial slides sold by Gerrard and Philip Harris. I also took some histology slides from Lambeth Hospital that I bought from Clarkson’s in High Holborn in 1963 when I was looking for my first microscope. I tried to include some unusual specimens, including grasshopper, guppy, ostrich, potato fungal rot, slime mould and swan mussel.

Stained sections
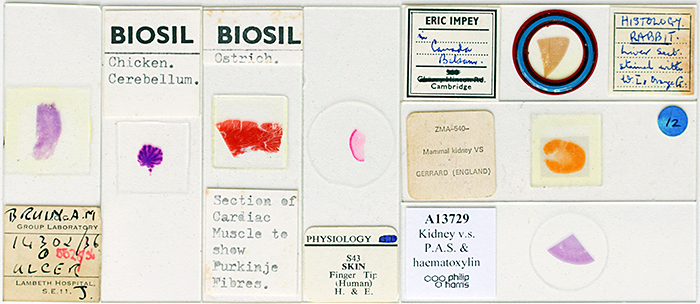
Stained sections
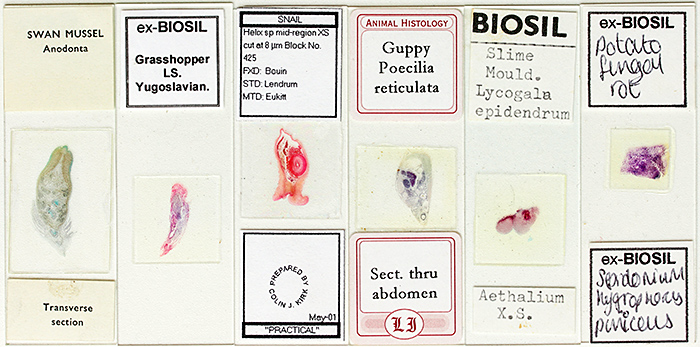
Stained sections
Later in the month, I went to Chinbrook Meadows for the event organised by the Friends as part of London Rivers Week. There is no electricity, so I borrowed my wife’s powerbank so that I could use an LED ring-light with my Olympus SZ4045 stereomicroscope. We found lots of specimens in the ponds in the new wetlands area, and also showed some of the plants from the banks of the ponds under microscopes. Back home, I photographed similar specimens to include in the meeting report. For the plants I used my Canon EOS 600D with a 60 mm f/2.8 EF-S macro lens, and for the hairs I used a 4× Olympus SPlan objective and an NFK 2.5 L photo eyepiece on my BHT.

Cleavers (Galium aparine) and its hooked leaf hairs
(the hooked hairs are about 0.13 mm long)

Wall barley (Hordeum murinum) spike and its barbed awns (hairs)
(the awns are about 0.2 mm diameter)
One youngster asked us to identify an insect, and it was a ladybird larva. I couldn’t get a good photo of it, so when I got home I found a similar larva on our climbing rose and photographed it using my Canon 60 mm f/2.8 EF-S macro lens.

Ladybird larva (9 mm long)
March 2025
For the Zoom gossip meeting “Your go-to equipment”, I showed the LED ring-light that I use with my stereomicroscope and with macro lenses, and explained how I produce shadowless and dark-ground illumination.
Later in the month I went to the Quekett Spring Sale near Reading. It is a long trip by public transport, about 3½ hours, but I managed to sell a few items. I only bought one item, a Carl Zeiss C5× eyepiece, that I intend to try out with a T-mount microscope adapter.
February 2025
Joan Bingley and I took stereomicroscopes and specimens to the “Life Under a Microscope” session of the Wimbledon Common Nature Club. It is run by Auriel Glanville and her assistants (Jen Long, Luci Teuma and Alexander and Oliver Mallett) and welcomes children from 6–14 years old to come and discover the world of nature on the Common. They meet for 2 hours each month in the Information Centre, the same venue as used by Quekett members on excursions, the Weekend of Nature and the Open Day. I took my Olympus SZ4045 stereomicroscope with an LED ring-light, and a small and simple stereomicroscope.

Families with my stereomicroscopes
Joan brought a small Brunel stereomicroscope with transmitted and reflected light, and an old Orion S420 with a torch as a light source.

Joan Bingley’s stereomicroscopes
The specimens that the children brought back from their walk were mostly lichens, but they also brought back holly leaves and bramble stems. I photographed these later to include in the meeting report.

Holly leaf spines

Bramble thorns
Later in the month, I went to the workshop on stains and staining in the Natural History Museum (led by Gordon Brown) and the Home Counties Meeting in Cobham (organised by Joan Bingley). My participation was limited to taking photographs and notes for meeting reports on the Quekett website.
Commenting on this blog
If you would like to comment on anything in this blog, please send me a message.