December 2015
It is only the first of December, but this is probably the only chance I will get this month to take a photograph.

Drupes (they are not berries) of European holly (Ilex aquifolium L.)
Canon EOS 600D, 60 mm EF/S macro lens at f/4.5, stack of 17 images in Zerene Stacker, lit by 2 desk lamps with a Kleenex diffuser
I am still busy checking the new website, and we are hoping it will go live before Christmas.
November 2015
Jellybean Creative Solutions are ready to start testing the new WordPress theme using the pages that I have prepared, so I have to stop working on the new website briefly. This has given me the chance to clean (breath plus a microfibre cloth) and examine the slides that I bought at Microscopium last month. A lot of them are ordinary, such as sections of leaves, stems and liver, but there are some unusual ones too. I have found an entire caterpillar, entire water boatman, tracheal system of a caterpillar, section of a tadpole, section of a rabbit embryo, and injections of intestines and lungs.

Iridescent dry-mounted legs of a fly from the west coast of Africa
Canon EOS 600D, 60 mm EF/S macro lens at f/4.5 with 8-dioptre close-up lens, stack of 7 images in Zerene Stacker, lit by 2 desk lamps with a Kleenex diffuser
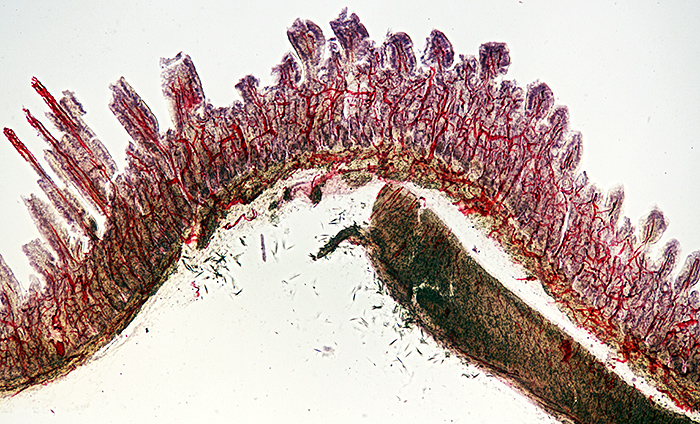
Injected and stained vertical section of the intestine of a cat
Nikon NA 0.85 achromatic condenser, Olympus SPlan 4× objective, NFK 2.5× photo eyepiece, stack of 9 images in Zerene Stacker
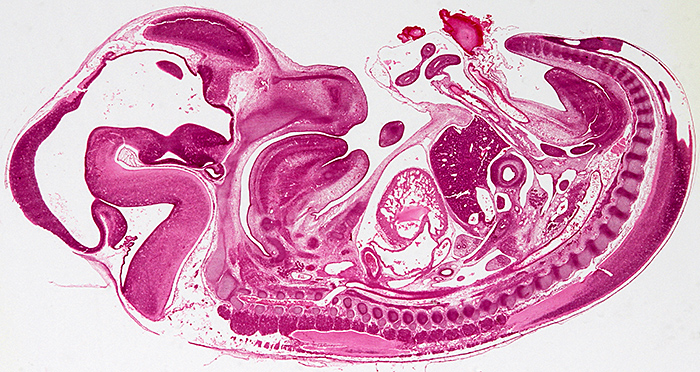
Longitudinal section of an entire rabbit embryo, slide by Flatters & Garnett
Canon EOS 600D, 60 mm EF/S macro lens at f/13, illuminated by a light box
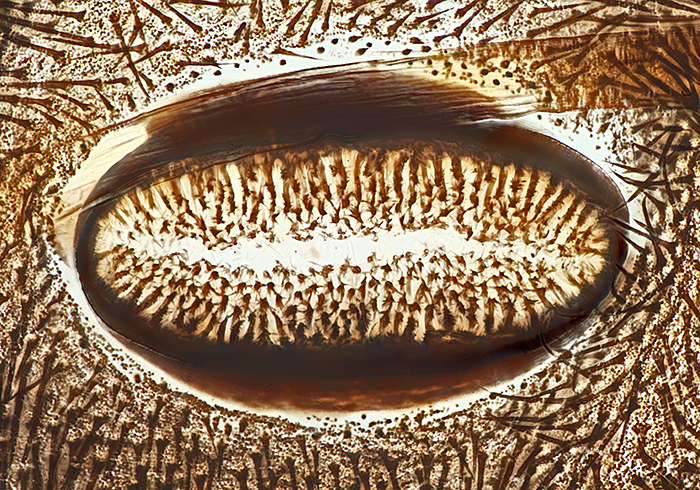
Spiracle of caterpillar of cinnabar moth (Tyria jacobaeae (L.)), slide by Eric Impey
Nikon NA 0.85 achromatic condenser, Olympus SPlan 40× objective, NFK 2.5× photo eyepiece, stack of 25 images at 2 µm intervals in Zerene Stacker
October 2015
I know I am supposed to be working flat out on the Club’s website, but I allow myself an occasional break to browse eBay, and I found a late version of the Olympus CK2 inverted microscope, the one with fine focus. Now I have to sell my old one.
I thought I was under pressure to get the new website ready for the end of March, but now Cubik Solutions have gone into liquidation and so their server with the Club website could be turned off at any time. We have found a company to design a WordPress theme for us, Jellybean Creative Solutions, and now we have to see how quickly they can provide a theme. I had made a small test site so that I could learn WordPress, but now it has got to become the new site and I am rushing to upload as much as I can from the old site.
At the Annual Exhibition, I showed examples of the 2 µm thin sections from Carolina Biological, with comparison photos with normal sections
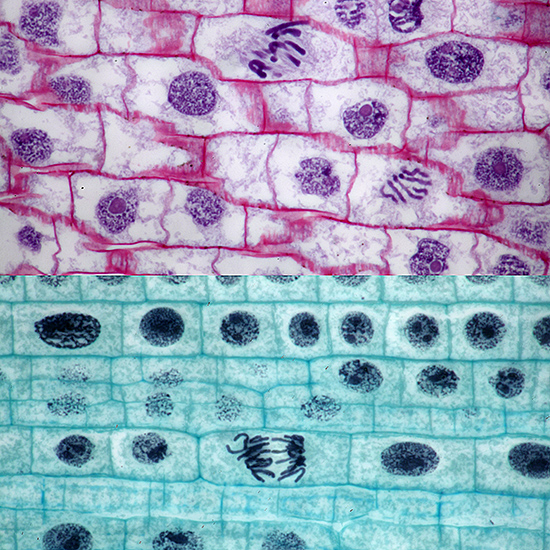
Mitosis in onion root, 2µm thin section at top
Olympus BH2-AAC condenser, SPlan 100× NA 0.95 dry objective, NFK 2.5× photo eyepiece, stacks of 5 and 8 images in Zerene Stacker
I didn’t get much time to stay near my exhibit, because I was busy trying to photograph everything and everyone, and lots of people wanted to talk about the website and about Olympus microscopes. My photo of a cypress seed cone received a Barnard Award.

Seed cone of Lawson’s cypress (Chamaecyparis lawsoniana (A. Murray) Parl.)
Canon EOS 600D with 60 mm EF-S macro lens + Olympus 13 cm supplementary lens, stack of 95 images in Zerene Stacker, lighting was 2 CF bulbs through a Kleenex diffuser. The cone is about 5mm wide.
Two weeks later was Microscopium at St Albans, so I took a day off from working on the website. I bought a bargain box of slides that seems to have some nice specimens, so I must find time to clean and photograph the best ones.
September 2015
I am not going to have much time for microscopy for the next 7 months; the Club’s website has turned out to be based on obsolete technology with no migration path to any other system, and the company that hosts it (Cubik Solutions) will not renew our annual contract at the end of March 2016. So I have to find a new hosting company (probably one that supports WordPress), specify and commission a new design, load all of the text and all of the images, add all of the internal page-to-page links, test everything, and make sure that it all works before the current site is turned off.
I managed to get 3 reasonable photographs ready to send to David Linstead for the Annual Exhibition next month, and spent 2 days on Quekett events. Saturday 12th was the autumn meeting at Penkridge, and Joan Bingley very kindly gave me a lift again. Ray Sloss and Mike Gibson were selling items that have been donated to the Club, including quite a lot of prepared slides, and I bought one of the human flea, Pulex irritans L.
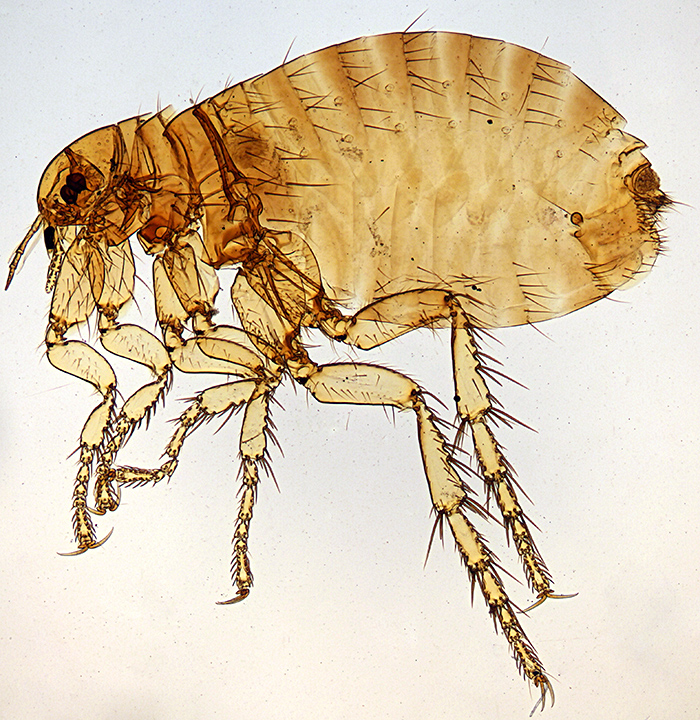
Female Pulex irritans L.
Olympus BH2-ULWD condenser, SPlan FL 2× objective and NFK 2.5× photo eyepiece, stack of 9 images in Zerene Stacker
The slide looks quite old but does not have the name of the preparer, and it appears to have been cut from window glass; it is not quite rectangular, measures about 79 × 27 mm and is a little over 2 mm thick.

Label data: “Flea Pulex irritans Female”
The next day was Open Day at Wimbledon Common, and Dennis Fullwood, Mary Morris, Barry Wendon and I put on a display in the Information Centre. There were lots of waterfleas in Queensmere, and I had a dish of them under my Olympus SZ4045 stereomicroscope, using a camera to display them on the Centre’s television so that they attracted attention from visitors. We also had a selection of galls on oak leaves and lichen on oak twigs.

Green lichen on oak twig
I must try to find a book to help me identify lichens, because I seem to photograph them quite often.
August 2015
People who have seen last month’s photo of Jamie Nelson’s laboratory have asked if I have anything similar. No, I don’t, because Kai and I live in a one-bedroomed flat with no space for a permanent setup. I use my microscopes on the dining table, a kitchen worktop or the desk in the bedroom, and I have to put away everything in cupboards when I have finished.
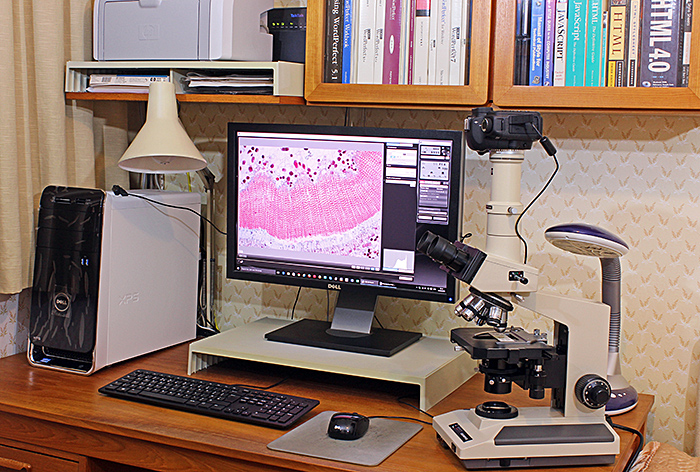
My setup for photomicrography
My trinocular Olympus BH-2 is at an odd angle so that I can use my right hand to turn the fine focus a known number of gradations and use my left hand to fire the camera using the mouse to click the button in EOS Utility. I can take a series of shots for stacking quite quickly with this arrangement.
Last year, I prepared comparison photos taken with 5 Olympus 10× objectives (E A, A, DPlan, SPlan and SPlan Apo) to assist an enquirer on amateurmicrography.net (Olympus LB Objective Question) and I was very disappointed with the performance of my SPlan 10×; viewed in EOS Utility on my computer screen there was no obvious point of sharpest focus and the photo lacked contrast. Under a magnifying glass the front element looked clean, so I have been looking for a replacement and today one appeared on eBay. Before clicking “Buy it now” I thought I should check the objective again, and this time I used my SZ4045 stereo and I could see a hazy film all over the front element. I managed to remove the film using isopropyl alcohol on Whatman 105 lens cleaning tissue. Then I got out my BH-2 and my DPlan and SPlan Apo 10× objectives and I was delighted to see that there is now a clear increase in image quality from DPlan to SPlan to SPlan Apo. I try to avoid cleaning objectives in case I damage them, but this time it really paid off.
I have tried photographing another subject for the 2015 annual exhibition, this time a seed cone of Lawson’s cypress (Chamaecyparis lawsoniana (A. Murray) Parl.)

Seed cone of Lawson’s cypress (Chamaecyparis lawsoniana (A. Murray) Parl.); the cone is 5mm wide. (Canon 60 mm EF/S macro lens at f/4.5 with 8-dioptre close-up lens, stack of 54 images in Zerene Stacker)
I am not really happy with this one either; some of the grey parts of the cone have lost all detail during the stacking, and there are stacking artefacts among the shoots. I think I need to get out my SZ4045 stereo microscope to help choose a different cone, and do a bit of pruning so that there are no overlapping shoots.
While I was looking at some waterfleas on the excursion to Warnham Local Nature Reserve, I noticed that they were active but not moving around, so I switched my EOS 600D from Live View to movie mode and made my first video:
Click the arrow at bottom left to watch the video (13 seconds)
Carl Zeiss Plan 2.5× objective with Olympus 2.5× NFK photo eyepiece
July 2015
At the gossip meeting on fossil slides last month, I was fascinated by the slides of plant fossils in coal, partly because it is amazing that plant tissues can be preserved so well and partly because of the skill needed to make a thin section of coal. I don’t buy many slides but I decided to try to find one of coal fossils, and now I have bought one from Richard Courtiour (eBay seller richieathome), a favourite seller with Quekett members who collect old slides.
 |
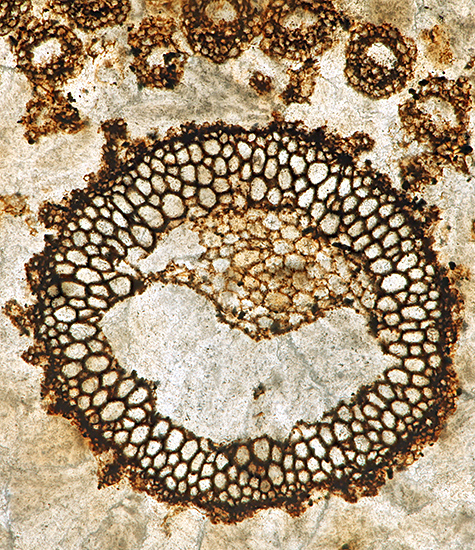 |
| Whole slide of Lepidostrobus oldhamius Williamson fossil in coal from Oldham | Photomicrograph of fossil in coal, Olympus SPlan Apo 10× objective and NFK 2.5× photo eyepiece, stack of 10 images in Zerene Stacker |
The slide is an unusual size, 76.5 × 30 mm, and the top surface is frosted, but the mountant makes the view clear through the coverslip.
The Quekett Microscopical Club tries to assist the families and executors of deceased members wanting to dispose of microscopes, accessories and slides, but this can be a considerable undertaking, involving collecting, storing, sorting, identifying, cleaning, photographing, cataloguing, auctioning, packing and despatching. We can only provide this service when members volunteer to help, and in recent years Phil Greaves has done most of the work. The executors of the estate of Dr Jamie Nelson (who died in March this year aged 101) recently offered his microscopes and related equipment to the Club, so Phil and I went to his home in Hampstead and collected several microscopes and several boxes of other items and loaded them into Phil’s Land Rover.

Part of Jamie Nelson’s home laboratory
Jamie was a very well known professional microscopist and was the managing director of McCrone Scientific Ltd in the UK. His home laboratory had been overflowing, with full shelves and cupboards around the walls, full tables in the middle, lots of items piled on the floor, metalworking facilities on one side, and a small darkroom off one corner. Jean Prentice and Sara Mark had already cleared a lot of stuff so that there was room to move around. Notable microscopical items included an Olympus BHC with objectives and condenser modified for Hoffman modulation contrast, 2 black Wild bright/dark field reflected-light attachments with the special objectives, and several stands that Jamie had modified for use in his experiments. Some of the equipment will probably be included in the Club’s 2016 members’ auction.
June 2015
At Warnham Local Nature Reserve last year, I showed some burrs of cleavers (Galium aparine L.) under my Olympus SZ4045 stereomicroscope with an LED ring light, and they looked so good that I made a mental note to take a photograph for the 2015 annual exhibition. This month I collected some cleavers during the Wimbledon Common BioBlitz and took them home to photograph.

Burrs of cleavers (Galium aparine L.) (Canon 60 mm EF/S macro lens at f/4.5, stack of 43 images in Zerene Stacker)
The photo is not bad, but I don’t like the burned-out highlights so I will have to experiment with a more diffused source of light. I am really impressed by my Canon EOS 600D camera; this photo is cropped and uses less than 25% of the width of the image. I also like the autofocus, and at Wimbledon I got reasonable shots of a caterpillar, a butterfly and a damselfly that I would not have even attempted with my Olympus OM 35 mm equipment.
The hooked hairs of cleavers are sometimes said to have been the inspiration for the Velcro® hook and loop fasteners (but the inventor said that he was inspired by cockleburs of Xanthium sp.), and Velcro® was one of the subjects that I took to Young Scientists’ Day in the Natural History Museum, the Quekett Microscopical Club’s annual outreach event aimed at introducing youngsters to microscopy.

Hooks of black Velcro® (Canon 60 mm EF/S macro lens at f/4.5, stack of 12 images in Zerene Stacker)
My favourite illuminator for my Olympus SZ4045 stereomicroscope is a Chinese ring light with 144 LEDs. It is nice and bright, it does not get in the way of the subject, and it provides good lighting for most subjects, for example the lichens in my May 2015 blog. I don’t often find an unsuitable subject, but some glossy black seeds at the Wimbledon Common BioBlitz gave horrible reflections:

Black, glossy seeds with white reflections from my LED ring light
The ring light has one weak point in its design – the black plastic ring for attaching it to the microscope has a 48 mm male thread that is not very strong, and mine has become very difficult to attach. Metal adapters are available from eBay seller bh542 in the USA but I have been reluctant to pay the high postage to the UK, and then a few days ago I found eBay seller microscoop-service in the Netherlands and bought 2 metal adapters from him, and they screw in nicely.
May 2015
Back in November 2013 at the annual exhibition of the British Entomological and Natural History Society at Kempton Park, Andy King, Dennis Fullwood, Joan Bingley and I were very impressed by the display of animal skeletons, feathers, nests and insect specimens by Susanna Ramsay. Many of the specimens were collected in Richmond Park (with permission, because it is an SSSI) and she takes them into primary schools in and around Kingston upon Thames to run workshops about local wildlife. We are hoping to arrange a joint event with Susanna, who has a website at Nature Collection, but we haven’t found the right opportunity yet. In November 2014, Susanna contacted us for advice on buying a trinocular stereomicroscope for use with her Nikon digital SLR, and David Linstead and Ray Sloss found a nice one with a pole stand and a very heavy base for her. She was happy with the microscope, but disappointed with the quality of her photos through the microscope, so Kai and I went to her house to see if we could help.
Sadly there was not much advice we could offer, and Susanna was already getting just about the best photographs that her stereomicroscope is capable of producing. Stereomicroscopes provide very nice visual images through the eyepieces, but the long working distance limits the numerical aperture, and photographs cannot match the quality of those taken with a macro lens or a compound microscope. We were able to show her how to increase the brightness of the live view image on the rear LED screen, and advise her that flat subjects such as butterfly wings are best tilted a little, not kept horizontal.
I haven’t bothered taking photographs with my Olympus SZ4045 stereomicroscope for several years, even though I have all the proper equipment. However, I do use it with my Canon EOS 5D Mark II for showing images on a television at events such as Wimbledon Common Stables Open Day, and it produces good images for that purpose. On the Wimbledon Common excursion this month, I also used this setup to photograph some specimens for the report, and I then decided to try some comparison shots.
All of the following photographs use the same white stage plate for the background, and the same 144 LED ring light for illumination, and were focused on a big computer screen using EOS Utility software. The photographs are not cropped – they show the whole image captured by the camera. The SZ4045 has the least contrast and the shallowest depth of field, and (of course) the stacked image has the greatest depth of field.

Foliose lichens on a pear twig (Olympus SZ4045 trinocular microscope, NFK 2.5× photo eyepiece)

Foliose lichens on a pear twig (Canon 60 mm EF/S macro lens at f/32, single shot)

Foliose lichens on a pear twig (Canon 60 mm EF/S macro lens at f/4.5, stack of 31 images in Zerene Stacker)
I am going to continue using a macro lens instead of a stereomicroscope whenever I can, and only use the stereomicroscope for photography when I have no alternative.
April 2015
I thought I knew all the local ponds after living in the same town for over 30 years, but I have found another one, and with lots of duckweed and decaying leaves it looks promising. It is in the park where I used to push my father in his wheelchair, so I don’t know how I overlooked it. Annoyingly, this month I am busy drawing chemical structures for a revised ISO standard so I probably won’t have time to go pond dipping.

Pond in Fishponds Park
People who read this blog, or have seen my website, or have seen my exhibits at Quekett meetings, sometimes ask why I am so keen on Olympus microscopes and Canon cameras. For me, it was an obvious choice. At home and at work, I used Olympus OM 35 mm cameras and I had a lot of their close-up and macro equipment, and Olympus produced adapters for using OM cameras on their microscopes, so when I decided that I wanted a modern microscope in preparation for retirement I did not even consider other makes of microscope. I am sure that the other major current microscope brands (Leica, Nikon and Zeiss) are just as good optically and mechanically as Olympus, and so are some of the older brands (AO, Leitz and Wild, for example) but so far I have not been tempted to change.
I expected to carry on using my OM cameras for many years, but digital SLRs improved much faster than I expected and became suitable for use on microscopes with the introduction of the Canon EOS 40D. I couldn’t resist the instant results and the ability to control the camera from my computer, and fortunately you only need a cheap and simple adapter to replace an OM body with an EOS. It would have been just as easy to use an Olympus digital SLR, but their sensors are small (resulting in a very restricted field of view when they are used to replace a 35 mm camera) and they lack Canon’s vibration-free mode and full computer control.
While returning the Club’s laptop, banner and books to the Natural History Museum after the Antique Scientific Instrument Fair, I had the opportunity to visit Dennis Fullwood’s workstation where he is restoring microscope slides of Collembola. On many of the slides, the specimen is acting as a nucleus for crystallisation of the mountant, so Dennis transfers the labels to a new slide, dissolves the old mountant, uses isopropyl alcohol to dehydrate the specimen, then transfers it to xylene before re-mounting using Canada balsam. Very impressive, but it is a slow process and there are 3 large cabinets of Collembola slides. Dennis also showed me the special diatom arrangement that Klaus Kemp has made for the Club’s 150th anniversary.
March 2015
I can’t normally get to Quekett meetings far from London, but Joan Bingley kindly gave me a lift to and from the West Midlands meeting in Penkridge. I hadn’t realised there would be so much for sale, but Paul Wheatley, Peter Evennett, Spike Walker, Les Franchi and others were offering all sorts of equipment and books at bargain prices and I failed to resist an England Finder Slide from Les. I have found the instructions on the Internet.

Close-up of an England Finder Slide (each of the 1809 squares is approximately 1 mm × 1 mm)
Olympus SPlan 4× objective and NFK 2.5× photo eyepiece, stack of 6 images in Zerene Stacker
I was pleasantly surprised to see Colin Kirk selling his beautifully-prepared slides for just £2 each, and I couldn’t resist buying a few.
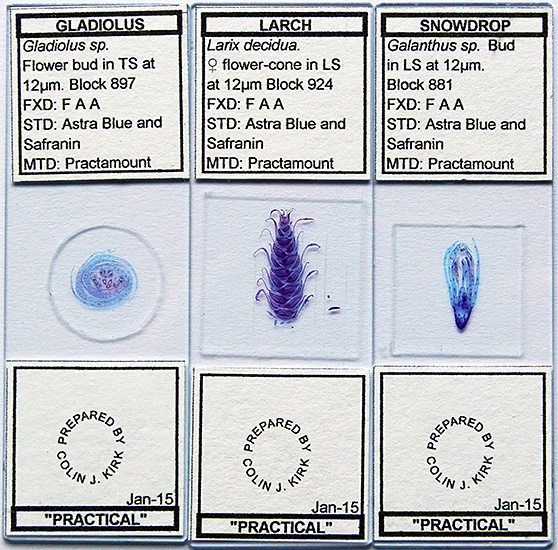
Three slides by Colin Kirk
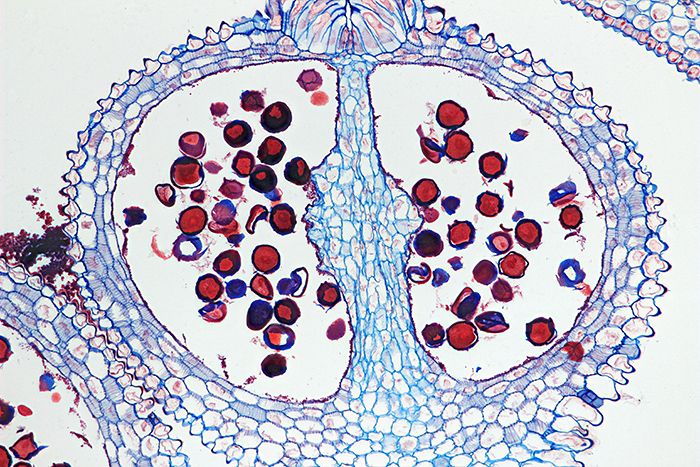
Transverse 12 µm section of Gladiolus flower bud by Colin Kirk, stained with Astra Blue and Safranin
Olympus SPlan Apo 10× objective and NFK 1.67× photo eyepiece, stack of 15 images in Zerene Stacker
I acquired a collection of Olympus brochures and price lists from Peter Evennett, several of which I had not seen before. I will add them to my Olympus microscope documentation page when I have time. I was particularly pleased to obtain a brochure for the IMT-2 inverted microscope because it included an item that I bought some time ago but have not been able to identify. It is a tube that is intended to replace a trinocular head but contains no lenses or prisms, so there is no glass between the NFK photo eyepiece and the sensor in a digital SLR, and the top has the circular dovetail for attaching a Photomicro Adapter L. It is quite substantial, weighing 458 grammes, 70 mm diameter and 135 mm tall. Now I know that it is an IMT2-PT Photo Tube. It fits in place of a binocular or trinocular head, and so it can also be used on my BH-2, CH-2 and CK2 microscopes.

Olympus IMT2-PT Photo Tube
February 2015
I am trying not to buy any more equipment but I couldn’t resist a CH2-FS attachment for converting my Olympus CH-2 microscope to Köhler illumination. It consists of 2 parts, a field iris diaphragm that sits on top of the light outlet in the base and an auxiliary lens that attaches to the bottom of the Abbe condenser. It should just work, but I couldn’t raise the condenser enough to bring the iris into focus. I lowered the condenser and could then see a bright metal rod that seemed to be an adjustable stop and what appeared to be a recessed set screw.
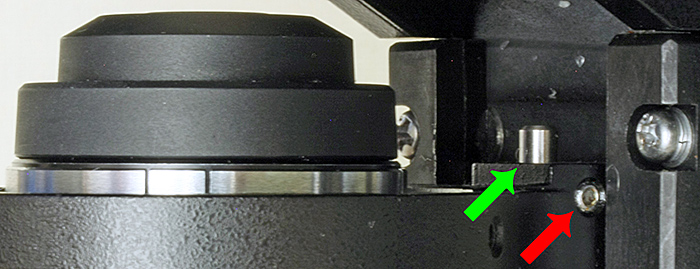
Substage in an Olympus CH-2 microscope – the top of the Abbe condenser is on the left, the green arrow shows the adjustable stop, and the red arrow shows the set screw
I tried to find an Allen key that would fit the screw but none of them would fit. I couldn’t get close enough to examine the screw with a magnifying glass so I used my SZ4045 stereomicroscope on a boom stand. This revealed a clear film over the screw and down into the recess, and I was able to scrape off the film with a pin. A 1.5 mm Allen key then fitted the screw, but when I loosened it the metal rod shot out because I hadn’t realised that there was a strong spring under it. Fortunately the spring stayed in place and I found the rod, and by a process of trial and error I secured it in a position that allows the field iris to be brought into focus.
Acording to the CHS instruction book, the CH2-FS attachment only works with objectives from 10× to 40×, but mine works fine with a DPlan 4× and an A 60× and there is only very slight cut-off with a Carl Zeiss Plan 2.5×, so I am very pleased with it.
January 2015
When Ernie Ives’ house was cleared last year, more than 30 copies of his self-published book A Guide to Wood Microtomy; Making quality microslides of wood sections came to light. The Quekett wanted to make these available for just the cost of postage and packing and I have been organising this. They have proved to be popular; after offering them on microscopy groups on the Internet they have all been distributed to microscopists around the world. Ernie produced a second edition in 2009 on CD; contact the webmaster if you would like a link to download the contents of the CD.
I only met Ernie once, at the 2012 Reading Convention, when I bought these 2 slides from him for £1 each. He took great care over the appearance of his slides as well as the quality of the sections, as you can see from the following photographs.

Slides made by Ernie Ives, with safranin-stained sections of Tectona grandis Lf. and Magnolia grandiflora L.
A photomicrograph of the lower right section of southern magnolia is shown below
Ernie used a consistent layout for the 3 wood sections on his slides; the large section on the left is transverse, top right is radial and bottom right is tangential. The red stain that he used was safranin.
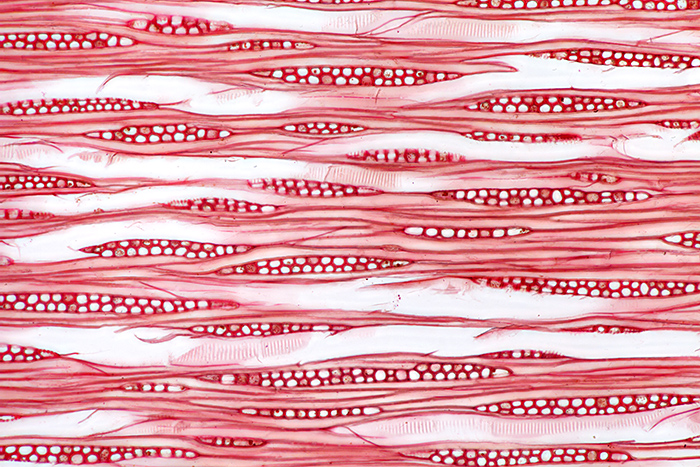
Safranin-stained tangential section of wood of Magnolia grandiflora L., from a slide prepared by Ernie Ives
Olympus SPlan Apo 10× objective and NFK 2.5× photo eyepiece, stack of 8 images in Zerene Stacker
Commenting on this blog
If you would like to comment on anything in this blog, please send me a message.